With vaccination, regular screening and treatment, cervical cancer is highly preventable, yet it is recognised as one of the top causes of death for women around the world. This preventable disease is far too common. Cervical cancer is missed and diagnosis is often delayed.
Significant strides have been made in decreasing cervical cancer rates around the world. In fact, Member States of the World Health Organization (WHO) have adopted a number of decisions to advance global public health, including specific goals calling for the elimination of cervical cancer.
Over the past several decades, screening strategies based on Pap cytology have saved millions of lives, but there is significant need for improvement. Abnormal Pap test results are common and women often endure repeat testing and waiting before they learn if cervical disease is present. Next generation screening strategies for screening, triage and diagnosis can help clinicians find and treat disease and to stop cancer from developing.
Triage and diagnosis of pre-cancers using biomarker technology simplifies testing and ensures laboratories and doctors get clear, actionable information. Based on more definitive risk assessment, women can be given the right guidance and care at the right time, protecting them from the potential harms or over- or under-treatment.
Learn about new new approaches in cervical cancer screening and management through our experts’ opinions:
- Slovenia: prof. Špela Smrkolj, PhD, MD, president of the Slovenian Society for gynaecologic oncology, colposcopy and cervical pathology and head of the Expert group for gynecology at National cervical cancer screening program will highlight advances in cervical cancer prevention strategies that are supporting the vision of cervical cancer elimination.
- Croatia: Prof.dr.sc. Herman Haller, president of Croatian Society for gynecologic oncology, prof.dr.sc. Jasmina Vraneš, head of the Reference Center of MoH for the diagnosis of sexually transmitted infections, doc.dr.sc. Danijela Vrdoljak-Mozetič, president of Croatian Society for Clinical Cytology and mr.sc. Renata Obrad-Sabljak, gynaecologist from Primary care centre Zagreb joint in multidisciplinary panel discussion on the topic of cervical cancer screening in Croatia.
HPV in primary screening
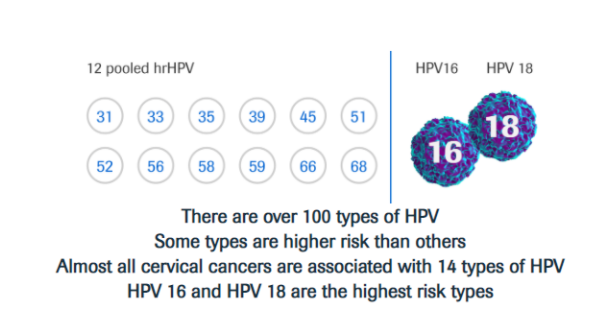
The discovery that infection with high-risk Human Papilloma Virus (HPV) is responsible for more than 99% of cervical cancer cases of cervical cancer (RMV) has led to the development of new, more sensitive screening HPV tests and HPV vaccines, which are used for primary prevention.
HPV is very common and most women will be exposed to it at some point in their lives. Most HPV infections go away on their own, without causing any problems. When an HPV infection persists over time, it can cause abnormal cellular changes that may lead to cervical precancer or cancer. It is important to identify women at risk with a reliable, sensitive test such as HPV DNA, and then assess their risk with a specific, reliable test to rule in or rule out disease.
Today, the use of HPV tests is included in many primary screening programs for cervical cancer in women >25 years old, based on convincing evidence of better sensitivity, reliability, and repeatability of results compared to the Pap test.
Studies have proven that primary HPV testing is more accurate than the Pap test and improves the assessment of a woman’s individual risk for developing cervical cancer. Many countries have already evolved to primary HPV screening with HPV DNA assay. Most professional medical societies and Ministries of Health are updating their country guidelines to include primary HPV testing.
Immunocytochemistry staining p16 / Ki-67 – an effective method of triage
The main advantage of the HPV test is high analytical sensitivity, but it detects many transient infections that can resolve spontaneously. Advanced biomarker–based testing for triage fills in the gaps by identifying clear evidence of cell transformation to precancer or cancer. Numerous studies have shown that the effectiveness of HPV screening can be increased by applying dual immunocytochemistry staining that looks for the simultaneous presence of two biomarkers – p16 and Ki-67 – in a cervical cytology sample. Therefore it detects high-grade intraepithelial lesions and provides definitive information to help differentiate which HPV positive women may benefit most from immediate intervention. Women who test negative on dual-stain show no signs of transforming infection and can be given more time to allow their body to clear the virus, without intervention. The test is performed on the same sample collected for a Pap or HPV test, eliminating the need for additional office visits or waiting time.
An extensive European EEMAPS study confirmed the high efficacy and specificity of p16 / Ki-67 immunocytochemistry staining for the identification of HSIL in triage, which could potentially lead to a reduction in unnecessary colposcopies. The study also found that the p16 / Ki-67 test is the only effective triage method for women with LSIL cytology results.
There has been good progress at protecting women from developing cervical cancer but we can all do better
With proper examination, vaccination, and treatment of precancer lesions, cervical cancer can be almost completely prevented.
New screening methods provide even more effective detection of women at highest risk for developing cervical cancer and those in need of urgent treatment due to present cervical changes. Therefore, with an optimistic view of the future, we can reasonably expect that women who have lost their lives due to cervical cancer will soon no longer have to be reported.
References:
- Bergeron, C., et alProspective evaluation of p16/Ki-67 dual-stained cytology for managing women with abnormal Papanicolaou cytology: PALMS study results. Cancer Cytopathology, 123(6), pp. 373-81.
- Castle, P.E., et al, 2011. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. The Lancet Oncology, 12(9), pp. 880-90.
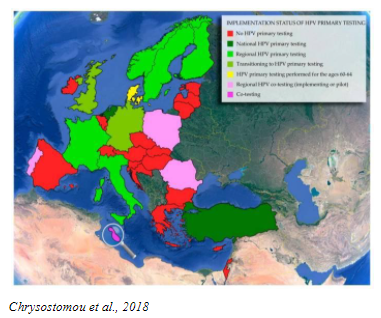
- Chrysostomou, C.A.,et al, 2018. Cervical Cancer Screening Programs in Europe: The Transition Towards HPV Vaccination and Population-Based HPV Testing. Viruses, 10(12), pp. 729-64.
- Clarke, M.A., Cheung, L.C., Castle, P.E., Schiffman, M., Tokugawa, D., Poitras, N., Lorey, T., Kinney, W. & Wentzensen, N., 2018. Five-Year Risk of Cervical Precancer Following p16/Ki-67 Dual-Stain Triage of HPV-PositiveWomen. JAMA Oncology, 5(2), pp. 181-6.
- Cuschieri, K., et al 2018. Eurogin roadmap 2017: triage strategies for the management of HPV-positive women in cervical screening programs. International Journal of Cancer, 143(4), pp. 735-45.
- Cuzick, J., et al, 2006. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. International Journal of Cancer, 119(5), pp. 1095-101.
- Klaes, R., Friedrich, T., Spitkovsky, D., Ridder, R., Rudy, W., Petry, U., Dallenbach-Hellweg, G., Schmidt, D. & von Knebel Doeberitz, M., 2001. Overexpression of p16INK4a as a specific marker for dysplasia and neoplastic epithelial cells of the cervix uteri. International Journal of Cancer, 92(2), pp. 276-84.
- Petry et al, 2011. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 Dual-stained cytology. Gynecologic Oncology, 121(3), pp. 505-9.
- Schmidt, D., Bergeron, C., Denton, K.J., Ridder, R. & European CINtec Cytology Study Group., 2011. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathology, 119(3), pp. 158-66.
- Wentzensen, N., Schiffman, M., Palmer, T. & Arbyn, M., 2016. Triage of HPV positive women in cervical cancer screening. Journal of Clinical Virology, 76(Suppl 1), pp. S49-S55.
- Wentzensen, N. & von Knebel Doeberitz, M., 2007. Biomarkers in cervical cancer screening. Disease Markers, 23(4), pp. 315-30.
- Wright, T.C., Stoler, M.H., Behrens, C.M., Sharma, A., Zhang, G. & Wright, T.L., 2015. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecologic Oncology, 136(2), pp. 189-97.
- Wright, T.C., Behrens, C.M., Ranger-Moore, J., Rehm, S., Sharma, A., Stoler, M.H. & Ridder, R., 2017. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: Results from a sub-study nested into the ATHENA trial. Gynecologic Oncology, 144(1), pp. 51-56.
- www.diagnostics.roche.com
For HCPs only
MC-HR-01712