A recent publication froma large European clinical study that included >40,000 women aged 25-46, was evaluated the accuracy of cytology, HPV E6/E7 mRNA and p16/Ki-67 as triage tests and the performance of these tests to predict CIN2+ regression and virus clearance at the 1 year time point.1
HPV DNA-positive women were triaged with cytology and tested for E6/E7 mRNA and p16/Ki-67. Cytology positive women were referred to colposcopy, and negatives were randomly assigned to immediate colposcopy or to 1-year HPV retesting. Lesions found within 24 months since recruitment were included.
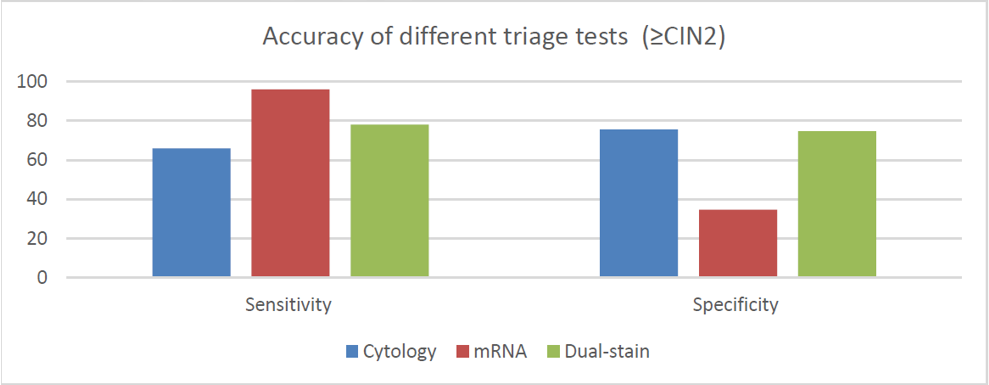
The results of this study clearly demonstrated good performance of p16/Ki-67 as a triage test:
- p16/Ki-67 showed higher sensitivity than cytology with similar colposcopy referral, even when keeping the same interval to retesting, leading to increased efficiency.
- E6/E7 mRNA assay has very high sensitivity but the immediate colposcopy referral rate was higher than the total referral with cytology, including those that may have regressed, negatively impacting specificity with this screening strategy
- Data from this study suggest that the p16/Ki-67 and E6/E7 mRNA biomarkers may predict CIN2+ clearance, which is also plausible given what we know about the molecular pathogenesis of the disease. The data clearly show that HPV infections in women negative to the p16/Ki-67 test or the E6/E7 mRNA assay have strongly increased probability of clearing within 12 months.
The data of this study adds to the other large clinical trials (PALMS,2 ATHENA PLUS,3 Kaiser NCI,4 Wolfsburg,5 and the IMPACT trial6) which unanimously support the clinical value of p16/Ki-67 triage for women who are at risk for cervical disease based on their cervical cancer screening results.
Publication can be accessed from the link below:
References:
[1] Rossi PG., et al. p16/ki67 and E6/E7 mRNA Accuracy and Prognostic Value in Triaging HPV DNA-Positive Women. JNCI J National Cancer Inst 2021.
[2] Bergeron C., et al., Prospective evaluation of p16/Ki-67 dual-stained cytology for managing women with abnormal Papanicolaou cytology: PALMS study results. Cancer Cytopathol. 2015
[3] Wright TC., et al., Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: Results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017
[4] Clarke MA., et al., Five-Year Risk of Cervical Precancer Following p16/Ki-67 Dual-Stain Triage of HPV-Positive Women. JAMA Oncology. 2019
[5] Petry KU., et al., Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 Dual-stained cytology. Gynecol Oncol. 2011
[6] 2 Wright TC., et al. Clinical Validation of p16/Ki-67 Dual-Stained Cytology Triage of HPV-positive women – Results from the IMPACT Trial. Intl J Cancer 2021
For HCPs only
MC-HR-01717