The Roche Cervical Cancer Portfolio provides three clinically validated tests to help identify women at risk and improve detection and confirmation of high-grade disease in a single round of screening. The powerful combination of these tests supports healthcare professionals in making decisions for their patients with confidence.
The landmark ATHENA clinical study helped shape our understanding of the role of high-risk HPV testing in cervical cancer screening. The ATHENA HPV trial1 was a large, prospective clinical study evaluating the performance of the cobas®️ HPV Test in three relevant populations: women with ASC-US cervical cytology, women with normal cervical cytology, and an overall screening population (25+ years) to explore HPV as a first-line test (ongoing longitudinal 3 year study).
ATHENA, with over 47,000 women enrolled, also set out to evaluate the medical value of testing for pooled hrHPV DNA as well as genotypes 16 and 18 individually.
ATHENA Plus Trial was a retrospective study comparing the results of CINtec® PLUS Cytology vs. Pap cytology triage of HPV (+) test results from a subset of samples collected in this trial. Results are correlated to histology follow-up reported in the ATHENA data.2
CINtec® PLUS Cytology outperforms Pap cytology as the triage test for HPV (+) screening results. Primary screening by the cobas® HPV DNA test with triage using the CINtec® PLUS Cytology test demonstrates high sensitivity and specificity to detect transforming HPV infections and helps avoid unnecessary colposcopy for women who do not need it.
The landmark IMPACT (IMproving Primary screening And Colposcopy Triage) trial was a cervical cancer screening study designed to clinically validate and support the FDA approval of tests in Roche Cervical Cancer Portfolio – CINtec® PLUS Cytology, a dual-stain biomarker-based test, and the cobas® HPV test for use on the cobas® 6800/8800 Systems. The multi-center, prospective clinical study included >35,000 women aged 25-65 undergoing routine screening.3
The results demonstrate that p16/Ki-67 dual-stained immunocytochemistry is safe and effective for the triage of HPV-positive women identified during primary HPV screening. It also offers an alternative to current triage strategies which are based on cytology, either alone or combined with HPV16/18 genotyping. 4
The CERTAIN (CERvical Tissue AdjunctIve aNalysis) study is one of the largest, most rigorous immunohistochemistry clinical studies. It analyzed the impact of adjunctive use of p16 IHC on diagnostic sensitivity and specificity for ≥CIN2 when p16 IHC was used according to the LAST recommendations.5
It showed that adjunctive use of p16 IHC provides more accurate and reproducible diagnostic results in the interpretation of cervical biopsies, ensuring that more patients are treated correctly without treating a larger number of patients. In LAST cases, diagnostic accuracy, diagnostic sensitivity, and diagnostic specificity were all significantly increased for ISPH&E + p16 compared to ISPH&E.
Guidelines
The International Agency for Research on Cancer (IARC) has developed supplements to the current European guidelines for quality assurance in cervical cancer screening. The supplements take into account the potential of primary testing for human papillomavirus (HPV) and vaccination against HPV infection to improve cervical cancer prevention and control.6, 7
Human papillomavirus (HPV) DNA testing is recommended in all resource settings.
The Roche Cervical Cancer Portfolio is covering the entire spectrum of screening, triage, and diagnostic solutions. It helps determining the individual level of risk a woman has so that you will know what to do next and when.
References:
1 Stoler MH., et al., High-risk human papillomavirus testing in women with ASC-US cytology: results from the ATHENA HPV study. Am J Clin Pathol. 2011
2 Wright TC., et al., Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: Results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017
3 Safaeian M., et al., The IMPACT trial: human papillomavirus, cervical cytology and histopathological results from the baseline and 1-year follow-up phase. J. Obstet. Gynecol. 2021
4 Wright TC., et al., Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: Results from the IMPACT trial. Int J Cancer. 2021
5 Stoler M.H., et al. Routine Use of Adjunctive p16 Immunohistochemistry Improves Diagnostic Agreement of Cervical Biopsy Interpretation: Results From the CERTAIN Study. Am J Surg Pathol. 2018
6 Huh WK et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. J Low Genit Tract Dis. 2015
7 von Karsa, L. et al. European guidelines for quality assurance in cervical cancer screening. Summary of the supplements on HPV screening and vaccination. Papillomavirus Research. 2015
For HCPs only
MC-HR-01718
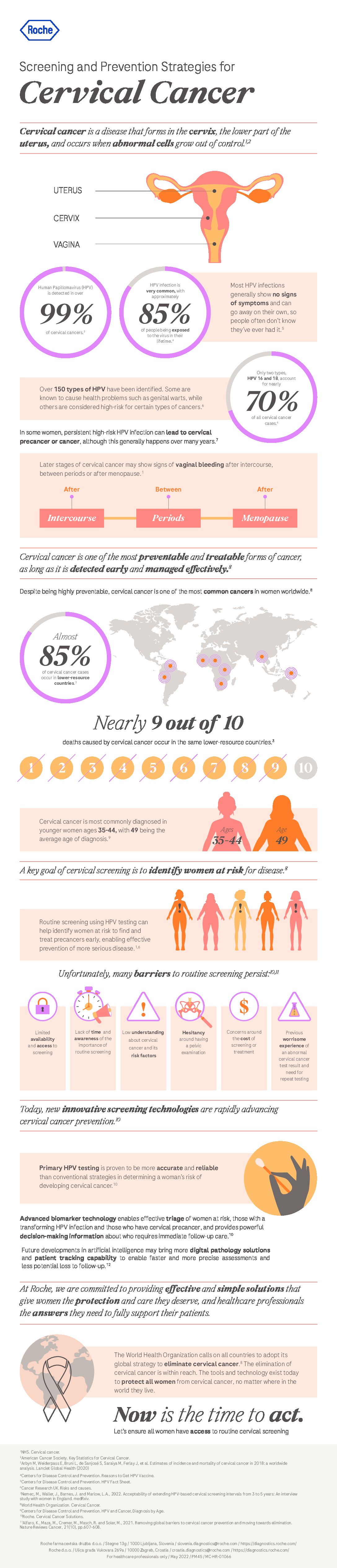
A recent publication froma large European clinical study that included >40,000 women aged 25-46, was evaluated the accuracy of cytology, HPV E6/E7 mRNA and p16/Ki-67 as triage tests and the performance of these tests to predict CIN2+ regression and virus clearance at the 1 year time point.1
HPV DNA-positive women were triaged with cytology and tested for E6/E7 mRNA and p16/Ki-67. Cytology positive women were referred to colposcopy, and negatives were randomly assigned to immediate colposcopy or to 1-year HPV retesting. Lesions found within 24 months since recruitment were included.
The results of this study clearly demonstrated good performance of p16/Ki-67 as a triage test:
- p16/Ki-67 showed higher sensitivity than cytology with similar colposcopy referral, even when keeping the same interval to retesting, leading to increased efficiency.
- E6/E7 mRNA assay has very high sensitivity but the immediate colposcopy referral rate was higher than the total referral with cytology, including those that may have regressed, negatively impacting specificity with this screening strategy
- Data from this study suggest that the p16/Ki-67 and E6/E7 mRNA biomarkers may predict CIN2+ clearance, which is also plausible given what we know about the molecular pathogenesis of the disease. The data clearly show that HPV infections in women negative to the p16/Ki-67 test or the E6/E7 mRNA assay have strongly increased probability of clearing within 12 months.
The data of this study adds to the other large clinical trials (PALMS,2 ATHENA PLUS,3 Kaiser NCI,4 Wolfsburg,5 and the IMPACT trial6) which unanimously support the clinical value of p16/Ki-67 triage for women who are at risk for cervical disease based on their cervical cancer screening results.
Publication can be accessed from the link below:
References:
[1] Rossi PG., et al. p16/ki67 and E6/E7 mRNA Accuracy and Prognostic Value in Triaging HPV DNA-Positive Women. JNCI J National Cancer Inst 2021.
[2] Bergeron C., et al., Prospective evaluation of p16/Ki-67 dual-stained cytology for managing women with abnormal Papanicolaou cytology: PALMS study results. Cancer Cytopathol. 2015
[3] Wright TC., et al., Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: Results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017
[4] Clarke MA., et al., Five-Year Risk of Cervical Precancer Following p16/Ki-67 Dual-Stain Triage of HPV-Positive Women. JAMA Oncology. 2019
[5] Petry KU., et al., Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 Dual-stained cytology. Gynecol Oncol. 2011
[6] 2 Wright TC., et al. Clinical Validation of p16/Ki-67 Dual-Stained Cytology Triage of HPV-positive women – Results from the IMPACT Trial. Intl J Cancer 2021
For HCPs only
MC-HR-01717
The landmark IMPACT (IMproving Primary screening And Colposcopy Triage) trial was a cervical cancer screening study designed to clinically validate and support the FDA approval of tests in Roche Cervical Cancer Portfolio – CINtec® PLUS Cytology, a dual-stain biomarker-based test, and the cobas® HPV test for use on the cobas® 6800/8800 Systems.
In May 2021 first results from the IMPACT study about the performance and safety of the cobas HPV on the cobas 6800/8800 Systems were published.1
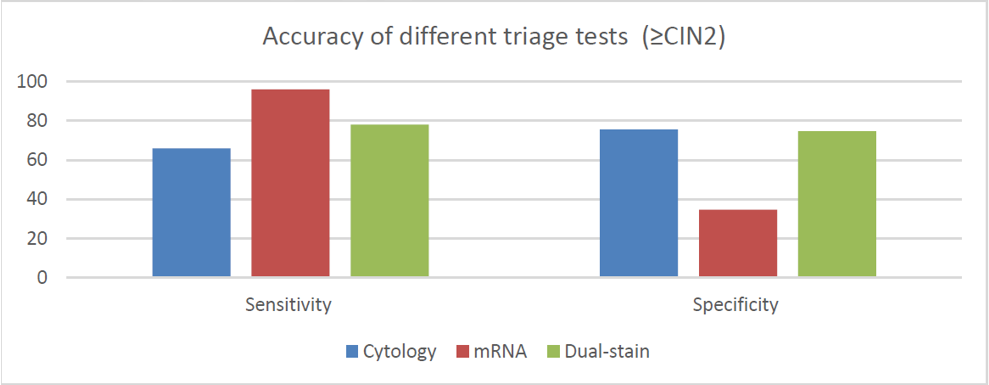
The aim of the recent publication from the IMPACT trial (IMproving Primary screening And Colposcopy Triage) was to evaluate clinical performance of p16/Ki-67 assay compared to triage using HPV16/18 genotyping combined with cervical cytology or cytology alone The multi-center, prospective clinical study included >35,000 women aged 25-65 undergoing routine screening.2
The study results demonstrated2:
- Higher sensitivity and efficiency when using only p16/Ki-67 assay for triage of primary HPV-positive women than Pap cytology-based triage for the detection of precancerous cervical lesions.
- Triage of HPV-positive results with p16/Ki-67 assay alone would have referred significantly fewer women to colposcopy than HPV16/18 genotyping with cytology triage of 12 “other” high-risk HPV genotypes.
- Triage with HPV16/18 genotyping with p16/Ki-67 assay for HPV-positive women provided significantly better sensitivity than triage with cytology alone or HPV16/18 with cytology.
- HPV-positive women with p16/Ki-67 negative test results showed a very low cumulative 1-year risk for disease which was significantly lower than the respective risks when using cytology with HPV16/18 genotyping, or cytology alone.
Performance of Dual-stain or cytology as a triage for HPV-positive results, or in combination with HPV 16/18 genotyping for detecting ≥CIN2 and ≥CIN3 in cobas 6800/8800 HPV-positive women with Dual-stain and cytology by HPV genotype: baseline data. CIN: Cervical intraepithelial neoplasia, DS: Dual stain, GT: genotyping, NPV: Negative predictive value, PPV: Positive predictive value
The authors are concluding that results from the study are demonstrating that p16/Ki-67 assay is safe and effective for the triage of HPV-positive women identified during primary HPV screening. p16/Ki-67-based triage provides consistently higher sensitivity than cytology-based triage, providing better reassurance against ≥CIN2 and ≥CIN3. Using DS alone as the triage reduces the complexity of triage strategies for HPV-positive women.
The IMPACT trial by Wright T., et al. can be accessed from the link below:
References:
[1] Safaeian M., et al.. The IMPACT trial: human papillomavirus, cervical cytology and histopathological results from the baseline and 1-year follow-up phase. J. Obstet. Gynecol. 2021
[2] Wright TC., et al. Clinical Validation of p16/Ki-67 Dual-Stained Cytology Triage of HPV-positive women – Results from the IMPACT Trial. Intl J Cancer 2021
For HCPs only
MC-HR-01716
The CERvical Tissue AdjunctIve aNalysis (CERTAIN) study was one of the largest immunohistochemistry (IHC) studies to date. It analyzed the impact of adjunctive use of p16 IHC on diagnostic sensitivity and specificity for ≥CIN2 when p16 IHC was used according to the LAST recommendations.1
The study set included 1.100 cervical biopsies representative of a U.S. colposcopy referral population. H&E-stained slides and p16 IHC-stained slides were prepared from each specimen. The participants in the study included 70 board-certified, individual surgical pathologists (ISPs) and 3 expert gynecological pathologists. Expert consensus diagnoses [Central Pathology Review using H&E alone (CPRH&E) and a second CPR using H&E + p16 IHC (CPRH&E+p16)] were established by the expert gynecological pathologists.1
Published results from the CERTAIN trial2 showed that:
- Adjunctive use of p16 IHC provides more accurate and reproducible diagnostic results in the interpretation of cervical biopsies, ensuring that more patients are treated correctly without treating a larger number of patients. In LAST cases, diagnostic accuracy, diagnostic sensitivity, and diagnostic specificity were all significantly increased for ISPH&E + p16 compared to ISPH&E.
- For cases for which pathologists requested p16 IHC according to LAST criteria, ISPs demonstrated a 10,4 % improvement in diagnostic agreement driven by an 11,8 % and 9,7 % increase in sensitivity and specificity, respectively.
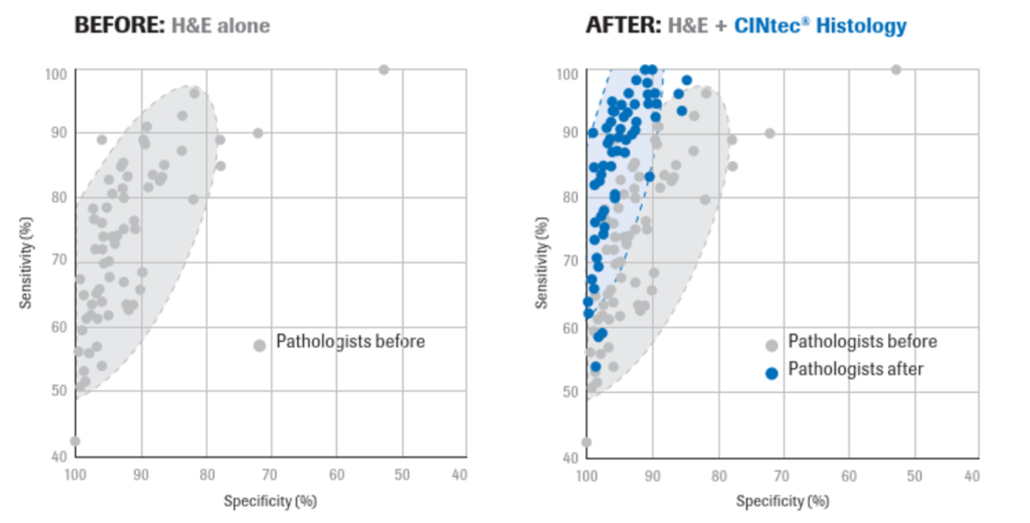
- In both LAST and non-LAST cases, p16 IHC did not lead to the over-diagnosis of p16 positive CIN1 as CIN2 or the under-diagnosis of p16 negative CIN2 as CIN1. When p16 IHC was performed according to LAST criteria, there were fewer ISP CIN2 diagnoses (n=2018) than when H&E was used alone (n=2524), reassuring that the use of p16 IHC does not lead to overdiagnosis of p16-positive CIN1 as CIN2. For non-LAST cases there was a comparable 11,0 % increase in sensitivity whereas specificity decreased slightly by -0,8 %.
The CERTAIN study by Wright T., et al. can be accessed from the link below:
https://pubmed.ncbi.nlm.nih.gov/29697437/
References:
[1] Stoler M.H., et al. Routine Use of Adjunctive p16 Immunohistochemistry Improves Diagnostic Agreement of Cervical Biopsy Interpretation: Results From the CERTAIN Study. Am J Surg Pathol. 2018
[2] Wright T.C., et al. The CERTAIN Study Results: Adjunctive p16 Immunohistochemistry Use in Cervical Biopsies According to LAST Criteria. Am J Surg Pathol 2021
For HCPs only
MC-HR-01715
The landmark IMPACT trial was a cervical cancer screening study designed to clinically validate and support the FDA approval of tests in Roche Cervical Cancer Portfolio – CINtec® PLUS Cytology, a dual-stain biomarker-based test, and the cobas® HPV test for use on the cobas® 6800/8800 Systems.
The IMPACT trial was a multi-center, prospective trial that included >35.000 women aged 25–65 years undergoing routine screening. It was designed to evaluate the performance and safety of the cobas HPV on the cobas 6800/8800 Systems, for triaging women with atypical squamous cells of undetermined significance (ASC-US), co-testing with Pap cytology, and primary HPV screening, and an immunocytochemistry-based dual stained cytology assay CINtec Plus Cytology for the detection of cervical epithelial cells simultaneously expressing the p16INK4a and Ki-67 biomarkers as a triage test for women with positive HPV test results.1
Published results from the IMPACT trial confirmed findings from previous studies2,3:
High sensitivity of cobas HPV to detect ≥CIN3 lesions
The 1-year follow-up results confirmed the continued high risk for ≥CIN3 among women who are cobas HPV-positive at baseline than among those with abnormal cytology. At the 1-year follow-up, 41 ≥CIN3 cases were diagnosed; 40 (97.6%) of these women were HPV-positive at baseline, whereas 21 (51.2%) had ≥ASC-US cytology at baseline.
Safety of HPV-negative result
The 1-year cumulative risk for ³CIN3 was 0.06% in HPV-negative women compared with 0.54% in cytology-negative women, demonstrating the superior protection provided by HPV testing compared with cytology. The very low baseline and 1-year risk of disease in HPV-negative women offers clinicians confidence in HPV-based cervical cancer screening.
Efficient risk stratification with genotyping
The risk for ≥CIN3 among women with HPV16 or HPV18 is high; after 1 year of follow-up, 1 in 3 HPV16-positive women at baseline, and 1 in 5 HPV18-positive women had ≥CIN3 diagnoses. The risk associated with being positive for any of the 12-Other HR-HPV genotypes was lower; baseline risk for ≥CIN3 among women with 12- Other HR-HPV genotypes was 3.3%, and 1-year CR was 5.3%. These findings are similar to those observed in ATHENA, where the baseline and 1-year CR for ≥CIN3 among women with 12-Other HR-HPV genotypes was 3.9% and 4.8%, respectively.2
First publication from IMPACT trial by Safaeian M., et al. can be accessed from the link below:
https://pubmed.ncbi.nlm.nih.gov/33852886/
References:
[1] Safaeian M., et al.. The IMPACT trial: human papillomavirus, cervical cytology and histopathological results from the baseline and 1-year follow-up phase. J. Obstet. Gynecol. 2021
[2] Wright TC., et al., The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012
[3] Wright TC., et al., Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: Results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017
For HCPs only
MC-HR-01714
With vaccination, regular screening and treatment, cervical cancer is highly preventable, yet it is recognised as one of the top causes of death for women around the world. This preventable disease is far too common. Cervical cancer is missed and diagnosis is often delayed.
Significant strides have been made in decreasing cervical cancer rates around the world. In fact, Member States of the World Health Organization (WHO) have adopted a number of decisions to advance global public health, including specific goals calling for the elimination of cervical cancer.
Over the past several decades, screening strategies based on Pap cytology have saved millions of lives, but there is significant need for improvement. Abnormal Pap test results are common and women often endure repeat testing and waiting before they learn if cervical disease is present. Next generation screening strategies for screening, triage and diagnosis can help clinicians find and treat disease and to stop cancer from developing.
Triage and diagnosis of pre-cancers using biomarker technology simplifies testing and ensures laboratories and doctors get clear, actionable information. Based on more definitive risk assessment, women can be given the right guidance and care at the right time, protecting them from the potential harms or over- or under-treatment.
Learn about new new approaches in cervical cancer screening and management through our experts’ opinions:
- Slovenia: prof. Špela Smrkolj, PhD, MD, president of the Slovenian Society for gynaecologic oncology, colposcopy and cervical pathology and head of the Expert group for gynecology at National cervical cancer screening program will highlight advances in cervical cancer prevention strategies that are supporting the vision of cervical cancer elimination.
- Croatia: Prof.dr.sc. Herman Haller, president of Croatian Society for gynecologic oncology, prof.dr.sc. Jasmina Vraneš, head of the Reference Center of MoH for the diagnosis of sexually transmitted infections, doc.dr.sc. Danijela Vrdoljak-Mozetič, president of Croatian Society for Clinical Cytology and mr.sc. Renata Obrad-Sabljak, gynaecologist from Primary care centre Zagreb joint in multidisciplinary panel discussion on the topic of cervical cancer screening in Croatia.
Panel discussion

HPV in primary screening
The discovery that infection with high-risk Human Papilloma Virus (HPV) is responsible for more than 99% of cervical cancer cases of cervical cancer (RMV) has led to the development of new, more sensitive screening HPV tests and HPV vaccines, which are used for primary prevention.
HPV is very common and most women will be exposed to it at some point in their lives. Most HPV infections go away on their own, without causing any problems. When an HPV infection persists over time, it can cause abnormal cellular changes that may lead to cervical precancer or cancer. It is important to identify women at risk with a reliable, sensitive test such as HPV DNA, and then assess their risk with a specific, reliable test to rule in or rule out disease.
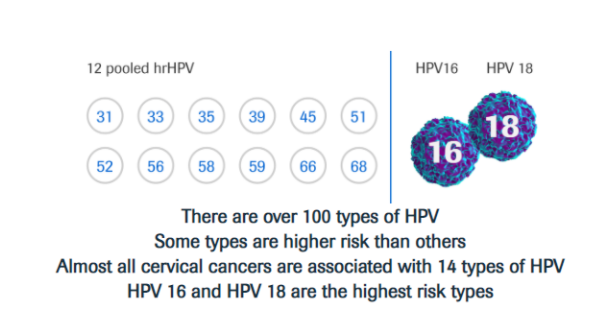
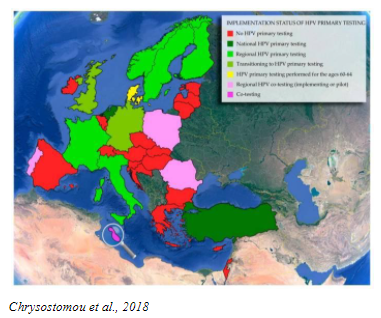
Today, the use of HPV tests is included in many primary screening programs for cervical cancer in women >25 years old, based on convincing evidence of better sensitivity, reliability, and repeatability of results compared to the Pap test.
Studies have proven that primary HPV testing is more accurate than the Pap test and improves the assessment of a woman’s individual risk for developing cervical cancer. Many countries have already evolved to primary HPV screening with HPV DNA assay. Most professional medical societies and Ministries of Health are updating their country guidelines to include primary HPV testing.
Immunocytochemistry staining p16 / Ki-67 – an effective method of triage
The main advantage of the HPV test is high analytical sensitivity, but it detects many transient infections that can resolve spontaneously. Advanced biomarker–based testing for triage fills in the gaps by identifying clear evidence of cell transformation to precancer or cancer. Numerous studies have shown that the effectiveness of HPV screening can be increased by applying dual immunocytochemistry staining that looks for the simultaneous presence of two biomarkers – p16 and Ki-67 – in a cervical cytology sample. Therefore it detects high-grade intraepithelial lesions and provides definitive information to help differentiate which HPV positive women may benefit most from immediate intervention. Women who test negative on dual-stain show no signs of transforming infection and can be given more time to allow their body to clear the virus, without intervention. The test is performed on the same sample collected for a Pap or HPV test, eliminating the need for additional office visits or waiting time.
An extensive European EEMAPS study confirmed the high efficacy and specificity of p16 / Ki-67 immunocytochemistry staining for the identification of HSIL in triage, which could potentially lead to a reduction in unnecessary colposcopies. The study also found that the p16 / Ki-67 test is the only effective triage method for women with LSIL cytology results.

There has been good progress at protecting women from developing cervical cancer but we can all do better
With proper examination, vaccination, and treatment of precancer lesions, cervical cancer can be almost completely prevented.
New screening methods provide even more effective detection of women at highest risk for developing cervical cancer and those in need of urgent treatment due to present cervical changes. Therefore, with an optimistic view of the future, we can reasonably expect that women who have lost their lives due to cervical cancer will soon no longer have to be reported.
References:
- Bergeron, C., et alProspective evaluation of p16/Ki-67 dual-stained cytology for managing women with abnormal Papanicolaou cytology: PALMS study results. Cancer Cytopathology, 123(6), pp. 373-81.
- Castle, P.E., et al, 2011. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. The Lancet Oncology, 12(9), pp. 880-90.
- Chrysostomou, C.A.,et al, 2018. Cervical Cancer Screening Programs in Europe: The Transition Towards HPV Vaccination and Population-Based HPV Testing. Viruses, 10(12), pp. 729-64.
- Clarke, M.A., Cheung, L.C., Castle, P.E., Schiffman, M., Tokugawa, D., Poitras, N., Lorey, T., Kinney, W. & Wentzensen, N., 2018. Five-Year Risk of Cervical Precancer Following p16/Ki-67 Dual-Stain Triage of HPV-PositiveWomen. JAMA Oncology, 5(2), pp. 181-6.
- Cuschieri, K., et al 2018. Eurogin roadmap 2017: triage strategies for the management of HPV-positive women in cervical screening programs. International Journal of Cancer, 143(4), pp. 735-45.
- Cuzick, J., et al, 2006. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. International Journal of Cancer, 119(5), pp. 1095-101.
- Klaes, R., Friedrich, T., Spitkovsky, D., Ridder, R., Rudy, W., Petry, U., Dallenbach-Hellweg, G., Schmidt, D. & von Knebel Doeberitz, M., 2001. Overexpression of p16INK4a as a specific marker for dysplasia and neoplastic epithelial cells of the cervix uteri. International Journal of Cancer, 92(2), pp. 276-84.
- Petry et al, 2011. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 Dual-stained cytology. Gynecologic Oncology, 121(3), pp. 505-9.
- Schmidt, D., Bergeron, C., Denton, K.J., Ridder, R. & European CINtec Cytology Study Group., 2011. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathology, 119(3), pp. 158-66.
- Wentzensen, N., Schiffman, M., Palmer, T. & Arbyn, M., 2016. Triage of HPV positive women in cervical cancer screening. Journal of Clinical Virology, 76(Suppl 1), pp. S49-S55.
- Wentzensen, N. & von Knebel Doeberitz, M., 2007. Biomarkers in cervical cancer screening. Disease Markers, 23(4), pp. 315-30.
- Wright, T.C., Stoler, M.H., Behrens, C.M., Sharma, A., Zhang, G. & Wright, T.L., 2015. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecologic Oncology, 136(2), pp. 189-97.
- Wright, T.C., Behrens, C.M., Ranger-Moore, J., Rehm, S., Sharma, A., Stoler, M.H. & Ridder, R., 2017. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: Results from a sub-study nested into the ATHENA trial. Gynecologic Oncology, 144(1), pp. 51-56.
- www.diagnostics.roche.com
For HCPs only
MC-HR-01712
Cervical cancer is a preventable disease. It is also curable if detected early and adequately treated. Yet it remains one of the most common cancers and causes of cancer-related death in women across the globe. The annual number of new cases of cervical cancer has been projected to increase from 570 000 to 700 000 between 2018 and 2030, with the annual number of deaths projected to increase from 311 000 to 400 000. More than 85% of those affected are young, undereducated women who live in the world’s poorest countries. Many are also mothers of young children whose survival is subsequently truncated by the premature death of their mothers (1).
Few diseases reflect global inequities as much as cancer of the cervix. In low- and middle-income countries its incidence is nearly twice as high and its death rates three times as high as in high-income countries. Proven and cost-effective measures for eliminating cervical cancer exist, but to date have not been widely implemented in regions of the world where the disease burden is highest. To be optimally effective, these measures must be scaled to national levels and delivered using health service platforms that are sensitive to women’s needs, their social circumstances, and the personal, cultural, social, structural and economic barriers hindering their access to health services. Health services that are integrated and people centered, and that respect and uphold women’s rights and dignity, are vital.
Urgent and bold action is needed to scale up and sustain implementation of the evidence-based interventions (human papillomavirus (HPV) vaccination, cervical cancer screening and management of detected disease) for eliminating cervical cancer as a public health problem, but such action must be strategic.
This global strategy to eliminate cervical cancer proposes:
- a vision of a world where cervical cancer is eliminated as a public health problem;
- a threshold of 4 per 100 000 women-years for elimination as a public health problem;
- the following 90-70-90 targets that must be met by 2030 for countries to be on the path towards cervical cancer elimination:
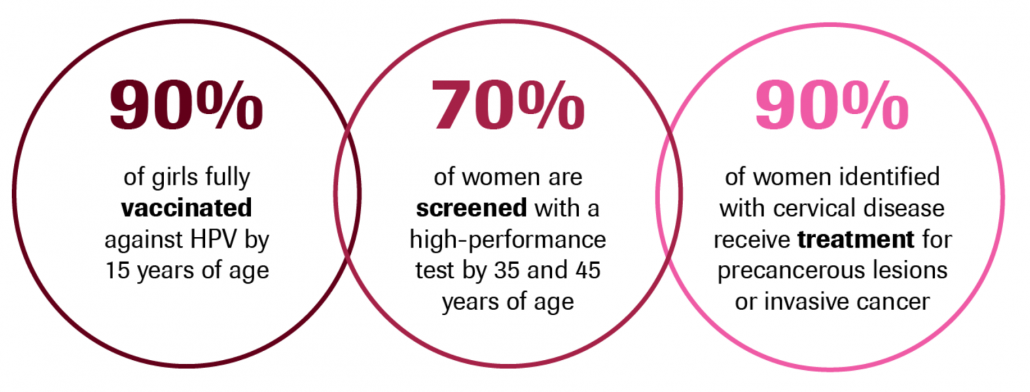
- a mathematical model that illustrates the following interim benefits of achieving the 90-70-90 targets by 2030 in low- and lower-middle-income countries:
- median cervical cancer incidence rate will fall by 42% by 2045, and by 97% by 2120, averting more than 74 million new cases of cervical cancer;
- median cumulative number of cervical cancer deaths averted will be 300 000 by 2030, over 14 million by 2070, and over 62 million by 2120.
The global strategy to eliminate cervical cancer as a public health problem will require:
- political support from international and local leaders;
- coordinated cooperation among multispectral partners;
- broad support for equitable access in the context of universal health coverage;
- effective resource mobilization;
- health system strengthening; and
- vigorous health promotion at all levels.
The interconnected nature of gender and health must stand as the strategic centerpiece of interventions. The strategy must also be open to the exploration and exploitation of new ideas and opportunities, including advances in developing new medicines, vaccines, diagnostics and treatment modalities. In order to achieve its targets, the strategy must embrace innovative models of service delivery and computerized data and information systems, together with new and expanded training methods (for example, using virtual reality simulations) and interventions scaled up to population level (for example, mass campaigns to screen and treat cervical cancer, and surgical camps). Management science and modern forms of communications technology must be integrated into all aspects of service delivery. The market must be reshaped to eliminate cost as a barrier to prevention and treatment in the world’s poorest countries.
The moment has arrived for an ambitious, concerted and inclusive strategy to accelerate eliminating cervical cancer as a public health problem. Elimination is within the reach of all countries. We know what works. The technology and tools exist. We know that prevention and early diagnosis and treatment are highly cost effective. The current focus on universal health coverage demonstrated by the United Nations General Assembly in September 2019 offers a unique opportunity for countries to strengthen interventions for the management of invasive cervical cancer (2).
“Through cost-effective, evidence-based interventions, including human papillomavirus vaccination of girls, screening and treatment of precancerous lesions, and improving access to diagnosis and treatment of invasive cancers, we can eliminate cervical cancer as a public health problem and make it a disease of the past.” Dr Tedros Adhanom Ghebreyesus, Director-General, World Health Organization
Please watch this video short of a full-length documentary on the global Conquering Cancer initiative. The video features inspirational stories from cervical cancer survivors and health care professionals who are looking at the 10-year horizon, and challenging country leaders to take action towards the goal of worldwide cervical cancer elimination by 2030.
More information:
WHO publication
Roche Global Access Program – Cervical Cancer
References:
- Mailhot Vega RB, Balogun OD, Ishaq OF, Bray F, Ginsburg O, Formenti SC. Estimating child mortality associated
with maternal mortality from breast and cervical cancer. Cancer. 2019;125(1):109–17. doi:10.1002/cncr.31780. - Resolution adopted by the General Assembly on 10 October 2019. Resolution 74/2: Political declaration of the highlevel meeting on universal health coverage. New York: United Nations General Assembly, Seventy-fourth session; 2019
(https://undocs.org/en/A/RES/74/2, accessed 2 October 2020).
For HCPs only
MC-HR-01710
Contact
Slovenia
Roche farmacevtska družba d.o.o.Stegne 13g
SI-1000 Ljubljana
Telephone: +386 1 360 26 00
e-mail: slovenija.info@roche.com
Croatia
Roche d.o.o.Ulica grada Vukovara 269a
10000 Zagreb
Telephone: +385 1 472 23 33
e-mail: croatia.info@roche.com
Disclaimer
This website contains information on products which is targeted to a wide range of audiences and could contain product details or information otherwise not accessible or valid in your country. Please be aware that we do not take any responsibility for accessing such information which may not comply with any legal process, regulation, registration or usage in the country of your origin.
This web page is not intended for reporting adverse drug reactions and other safety information.
Slovenia
Please report the suspected adverse reactions that you notice during drug therapy in accordance with the Rules on the Pharmacovigilance of medicinal products for human use (Official Gazette of the Republic of Slovenia, No. 57/14 and 27/17), in the way it is published on the website www.jazmp.si.
Roche contacts for collecting safety information regarding Roche medicinal products: slovenia.drugsafety@roche.com.
Croatia
Please report the suspected adverse reactions that you notice during drug therapy in accordance with the Ordinance on Monitoring Adverse Incidents Related to Medical Devices (Official Gazette, no. 125/13) in the way it is published on the website http://www.halmed.hr/
Roche contacts for collecting safety information regarding Roche medicinal products: croatia.info.ci1@roche.com.
Bosnia and Herzegovina
Please report the suspected adverse reactions that you notice during drug therapy in accordance with the Ordinance On Advertising Of Medicinal Products And Medical Devices (Official Gazette, no. 58/12) in the way it is published on the website http://www.almbih.gov.ba/
Roche contacts for collecting safety information regarding Roche medicinal products: bosnia.drugsafety@roche.com.