The landmark IMPACT (IMproving Primary screening And Colposcopy Triage) trial was a cervical cancer screening study designed to clinically validate and support the FDA approval of tests in Roche Cervical Cancer Portfolio – CINtec® PLUS Cytology, a dual-stain biomarker-based test, and the cobas® HPV test for use on the cobas® 6800/8800 Systems.
In May 2021 first results from the IMPACT study about the performance and safety of the cobas HPV on the cobas 6800/8800 Systems were published.1
The aim of the recent publication from the IMPACT trial (IMproving Primary screening And Colposcopy Triage) was to evaluate clinical performance of p16/Ki-67 assay compared to triage using HPV16/18 genotyping combined with cervical cytology or cytology alone The multi-center, prospective clinical study included >35,000 women aged 25-65 undergoing routine screening.2
The study results demonstrated2:
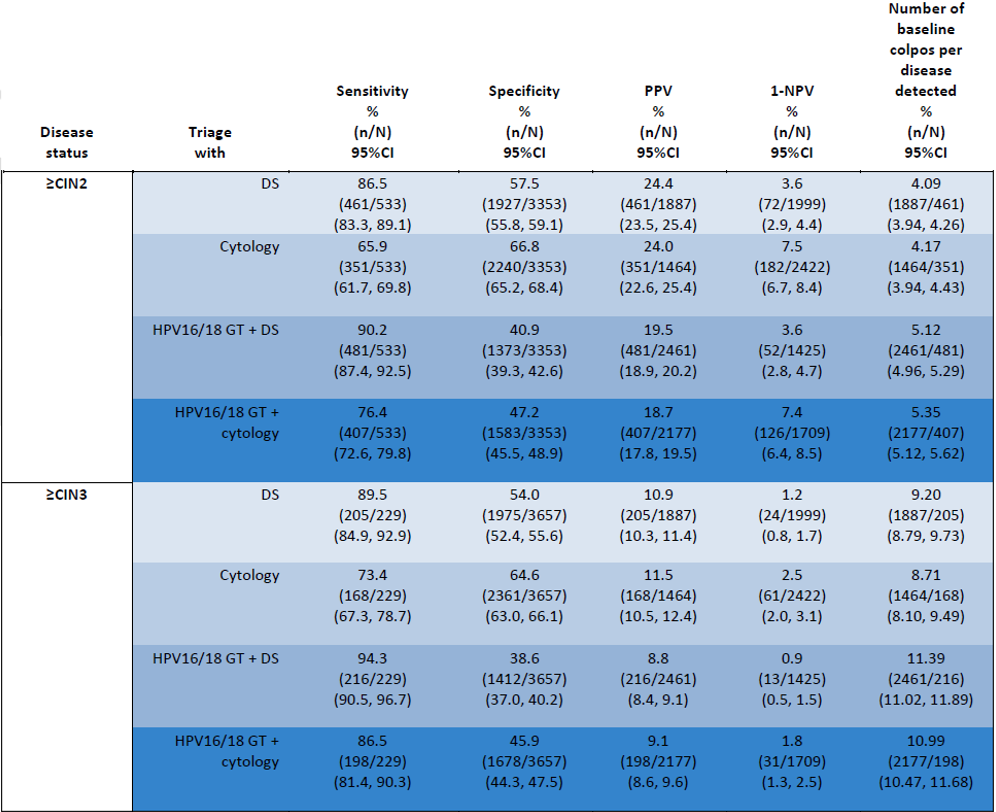
- Higher sensitivity and efficiency when using only p16/Ki-67 assay for triage of primary HPV-positive women than Pap cytology-based triage for the detection of precancerous cervical lesions.
- Triage of HPV-positive results with p16/Ki-67 assay alone would have referred significantly fewer women to colposcopy than HPV16/18 genotyping with cytology triage of 12 “other” high-risk HPV genotypes.
- Triage with HPV16/18 genotyping with p16/Ki-67 assay for HPV-positive women provided significantly better sensitivity than triage with cytology alone or HPV16/18 with cytology.
- HPV-positive women with p16/Ki-67 negative test results showed a very low cumulative 1-year risk for disease which was significantly lower than the respective risks when using cytology with HPV16/18 genotyping, or cytology alone.
Performance of Dual-stain or cytology as a triage for HPV-positive results, or in combination with HPV 16/18 genotyping for detecting ≥CIN2 and ≥CIN3 in cobas 6800/8800 HPV-positive women with Dual-stain and cytology by HPV genotype: baseline data. CIN: Cervical intraepithelial neoplasia, DS: Dual stain, GT: genotyping, NPV: Negative predictive value, PPV: Positive predictive value
The authors are concluding that results from the study are demonstrating that p16/Ki-67 assay is safe and effective for the triage of HPV-positive women identified during primary HPV screening. p16/Ki-67-based triage provides consistently higher sensitivity than cytology-based triage, providing better reassurance against ≥CIN2 and ≥CIN3. Using DS alone as the triage reduces the complexity of triage strategies for HPV-positive women.
The IMPACT trial by Wright T., et al. can be accessed from the link below:
References:
[1] Safaeian M., et al.. The IMPACT trial: human papillomavirus, cervical cytology and histopathological results from the baseline and 1-year follow-up phase. J. Obstet. Gynecol. 2021
[2] Wright TC., et al. Clinical Validation of p16/Ki-67 Dual-Stained Cytology Triage of HPV-positive women – Results from the IMPACT Trial. Intl J Cancer 2021
For HCPs only
MC-HR-01716